
A Carboxylic Acid is an organic compound containing a carboxyl functional group. They occur widely in nature and are also synthetically manufactured by humans. Upon deprotonation, carboxylic acids yield a carboxylate anion with the general formula R-COO – , which can form a variety of useful salts such as soaps.
The carboxylic acids are the most important functional group that present C=O. This type of organic compounds can be obtained by different routes, some carboxylic acids, such as citric acid, lactic acid or fumaric acid are produced by fermentation, most of these types of carboxylic acids are applied in the food industry.
The general formula of a carboxylic acid is R-COOH, where COOH refers to the carboxyl group, and R refers to the rest of the molecule to which this group is attached. In this carboxyl group, there exists a carbon which shares a double bond with an oxygen atom and a single bond with a hydroxyl group.
A carboxylic acid’s general formula is R-COOH, where COOH denotes the carboxyl group and R denotes the remainder of the molecule to which this group is linked. There is a carbon in this carboxyl group that has a double connection with an oxygen atom and a single bond with a hydroxyl group.
The first four carboxylic acids derived from alkanes are methanoic acid (HCOOH), ethanoic acid (CH3COOH), propanoic acid (C2H5COOH), and butanoic acid (C3H7COOH).
The general structure of a carboxylic acid is illustrated below.

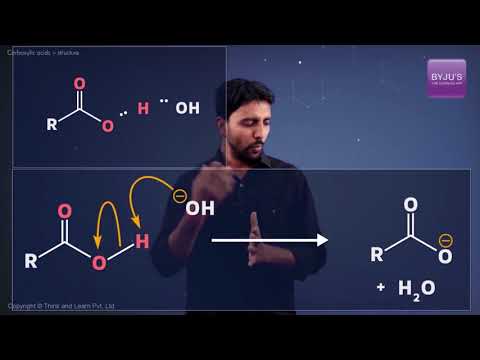
From the illustration provided above, it can be observed that a carboxylic acid contains a hydroxyl group attached to a carbonyl carbon. Due to the electronegativity of the oxygen atom, this functional group can undergo ionization and discharge a proton.
The carboxylate ion, produced from the removal of a proton from the carboxyl group, is stabilized by the presence of two oxygen atoms (through which the negative charge can move). Some common examples of carboxylic acids include acetic acid (a component of vinegar) and Formic acid.
The acidity of the carboxylic acid is explained in the video.
Generally, these organic compounds are referred to by their trivial names, which contain the suffix “-ic acid”. An example of a trivial name for a carboxylic acid is acetic acid (CH3COOH). In the IUPAC nomenclature of these compounds, the suffix “-oic acid” is assigned.
The guidelines that must be followed in the IUPAC nomenclature of carboxylic acids are listed below.
Some examples describing the nomenclature of carboxylic acids as per IUPAC guidelines are provided below.
| Trivial Name and Formula | IUPAC Name of the Carboxylic Acid |
| Formic acid, H-COOH | Methanoic acid |
| Crotonic acid, CH3CH=CH-COOH | But-2-enoic acid |
| Carbonic acid, OH-COOH | Carbonic acid |
| Butyric acid, CH3(CH2)2COOH | Butanoic acid |
Most of the properties of carboxylic acids are a result of the presence of the carboxyl group. Some physical and chemical properties of these compounds are discussed in this subsection.
In the manufacture of polymers, biopolymers, coatings, adhesives, and prescription products, carboxylic acids and their derivatives are used. They can also be used as solvents, antimicrobials, food additives, and flavourings.
Carboxylic acids are hydrocarbon compounds in which a carboxyl group has substituted one or more of the hydrogen atoms in the hydrocarbon. Methanoic acid (HCOOH), ethanoic acid (CH3COOH), propanoic acid (C2H5COOH), and butanoic acid (C3H7COOH) are the first four carboxyl acids derived from alkanes.
In plants and animals, certain carboxylic acids exist naturally. There is citric acid in citrus fruits, such as oranges and lemons. A large carboxylic acid with three ionizable hydrogen atoms is citric acid. It is present in citrus fruits and provides them with a sour or tart taste.
The carboxylic acids are acidic because of the hydrogen in the -COOH group, using the idea of an acid as a “substance that donates protons (hydrogen ions) to other things.” A hydrogen ion is moved from the -COOH group onto a water molecule in a water solution.
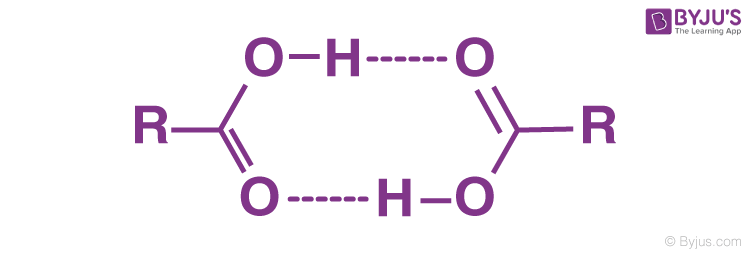
Carboxylic acids are defined as weak acids, meaning that in a neutral aqueous solution, they do not fully dissociate to create H + cations. Hydrogen bonds are formed between the individual molecules of the acid and water molecules. That’s why They partially ionise to give H + and RCOO − . Therefore they are called weak acids.
Thus, the general formula, structure, nomenclature, properties, and uses of carboxylic acids are briefly discussed. To learn more about these compounds and other types of organic compounds, such as aldehydes and ketones, register with BYJU’S and download the mobile application on your smartphone.
Test your knowledge on carboxylic acid properties

Put your understanding of this concept to test by answering a few MCQs. Click ‘Start Quiz’ to begin!
Select the correct answer and click on the “Finish” button
Check your score and answers at the end of the quiz
Visit BYJU’S for all Chemistry related queries and study materials
Your result is as below